This two-part penny change experiment uses vinegar, a household acid, to clean copper pennies. Kids can observe the oxidation process as the copper in the pennies reacts with oxygen in the air to form copper oxide and then malachite. This easy chemistry project gets kids interested in STEM → science, technology, engineering, and math!
Penny Change Science Experiment
Make dull pennies shine & turn green with this clever chemistry experiment!
Science Lab Supplies
- Free printable lab notebook with full instructions (get the free lab notebook here)
The lab book contains three easy science experiments for kids and contains areas to record your notes and observations. You can print it on card stock or plain paper and assemble it with ring clips or staples. It would be perfect for a science party.

Experiment Supplies
- 3 dull or dirty-looking pennies
- 1/4 cup white vinegar
- 1 teaspoon table salt
- paper towel or cotton pad
- small non-metal bowl
- Small non-metal container with a lid

Experiment 1 Instructions
Experiment One – Dull to Shiny
- Pour the vinegar and salt into the small non-metal bowl and stir to dissolve.

- Put 3 pennies into the bowl for about 30 seconds.

- The pennies are now shiny and look new! Take out the pennies and rinse them with fresh water. Place them on a paper towel to dry off.

Experiment 2 Instructions
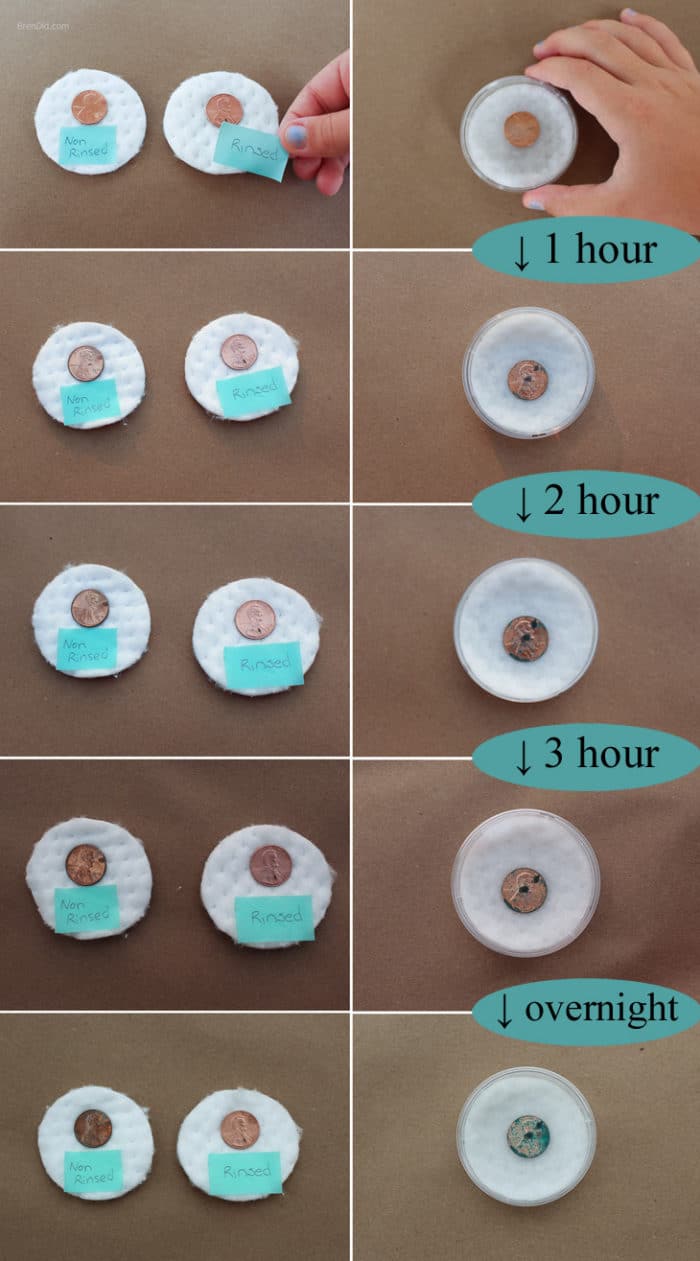
Experiment Two – Shiny to Green
- Fold a paper towel or cotton pad to fit the bottom of your container with a lid.
- Wet the paper towel or cotton pad with a few drops of vinegar.
- Put one penny on the wet pad and cover it with a lid. (The lid keeps the vinegar from drying out.)

- Put one penny on a dry pad or paper towel.
- Dip one penny in your salt/vinegar mixture and place it on a dry pad or paper towel.
- Observe your pennies at 1, 2, 3, and 8 hours.
Experiment Tip
Pennies made in 1982 or earlier are made of solid copper, use them in this reaction to make it work even better! Pennies made after 1982 are made from copper and zinc, they react a little less.
Experiment Follow-Up
Complete the lab notes section of the free printable lab notebook (available in the free printable library) with your kids. Have them record their predictions and observations. Which pennies oxidized the most quickly? Why do you think this happened? Look at the back of the penny on the vinegar pad. What does it look like? Why?

Experiment Explanation
Dull to Shiny – Pennies are made of copper or copper & zinc. They look dull or dirty when covered with copper oxide. Copper oxide forms when the copper reacts with oxygen in the air.
Vinegar is acetic acid. The acetic acid reacts with the salt (sodium chloride) to produce sodium acetate & hydrogen chloride. These quickly remove the copper oxide from the pennies, leaving a pure and shiny metal surface.
Shiny to Green – Shiny pennies have exposed copper to react with oxygen in the air to form copper oxide, making the pennies look dirty again. As the pennies continue to react with the oxygen, a green-blue compound called malachite forms.
Chemistry in Daily Life
The Statue of Liberty is covered with copper. Copper reacts with oxygen to form copper oxide and then malachite. The Statue of Liberty is green because of oxidation.
More Cool Science Experiments for Kids

Make sure to check out my post about Cool Science Experiments for Kids to download the free lab notebook and check out my Glitter Tornado & Fishing for Ice projects.
Like my free printables? Sign up for my newsletter below and never miss a thing!






Ely
What is the hypothesis of this experiment?
Bren
One possible hypothesis: If I expose clean pennies to oxygen in the air, then they will oxidize because they contain copper.
Another possible hypothesis: If I expose pennies to an acidic substance such as vinegar, the acid will clean off the tarnish or copper oxide.
Here are some great tips to write your own: https://www.sciencebuddies.org/blog/a-strong-hypothesis
Tina-ree Ferguson
very frustrated. I am trying to find the download for the penny lab notebook experiments and am going around in circles on your site trying to find it. Any help would be appreciated.
Bren
Tina, it’s on the free printables page just scroll down to the Craft Printables section.
Suhi
Thanks, my 7 year old son enjoyed this! nice to have a fun experiment that required so little supplies and he was excited to check on the pennies at each interval! Thanks so much!
Bren
Thanks, Suhi! I’m glad your family enjoyed the experiment!
Johannah
Can you put more than three at once, and can you do this with other things that are solid copper?
Bren
You can add as many pennies as you like, though it may slow the reaction time. I have not tried this with other metals. Let me know if you do!
Kim
What’s the real world application for this experiment?
Bren
Kim, the experiment shows how tarnish forms on copper items (like the Statue of Liberty or copper pans) and how the tarnish can be cleaned away.
Anonymous
Will it still work with Apple Cider Vinegar?
Bren
Yes, it will still work because apple cider vinegar contains acetic acid.
Kaylee
How long do I keep the 2 pennies on the vinegar pad?
Bren
As long as you desire!
Amber Massengill
Hi. I’m trying to find the free printable for the penny experiments. Is it still on your site and available to print?
Bren
Yes, it’s in the free printables library.